Modern eCOA Solution for Faster Studies
With our eCOA solution at the core, sponsors and CROs rely on uMotif’s scalable and configurable platform to deliver critical data capture.

Patient-centric solutions for faster, higher quality clinical trials and real-world studies.
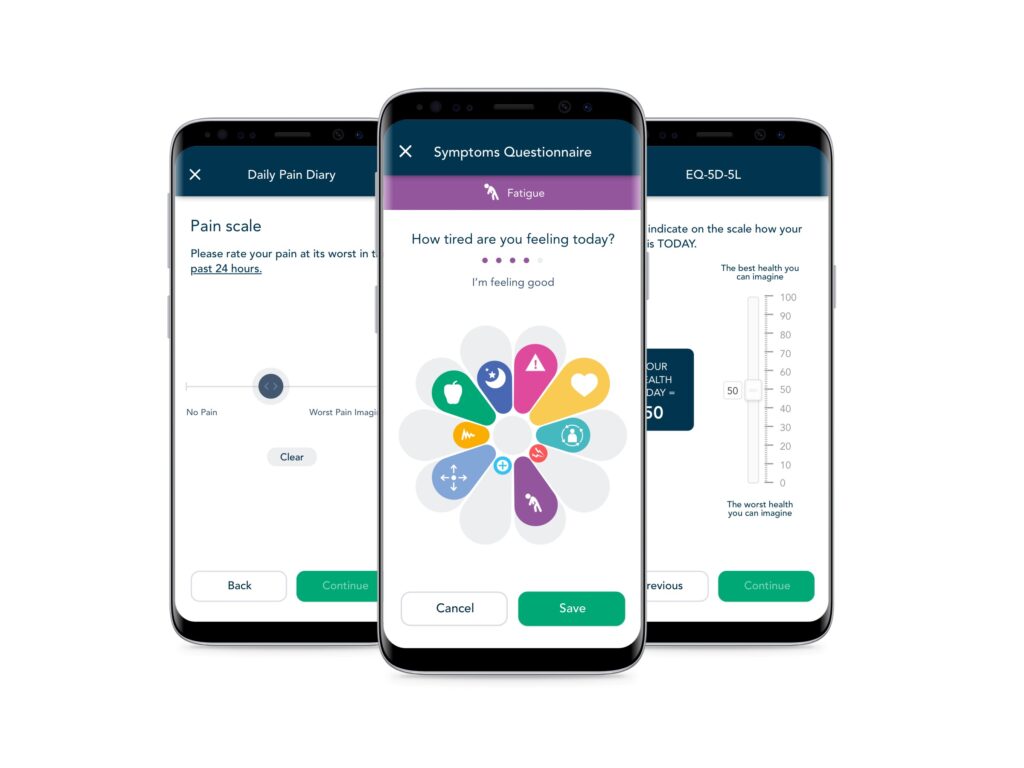
eCOA/ePRO and eDiaries
Quickly build and deploy validated instruments and study-specific diaries, across mobile and web applications, in any language, on any device.
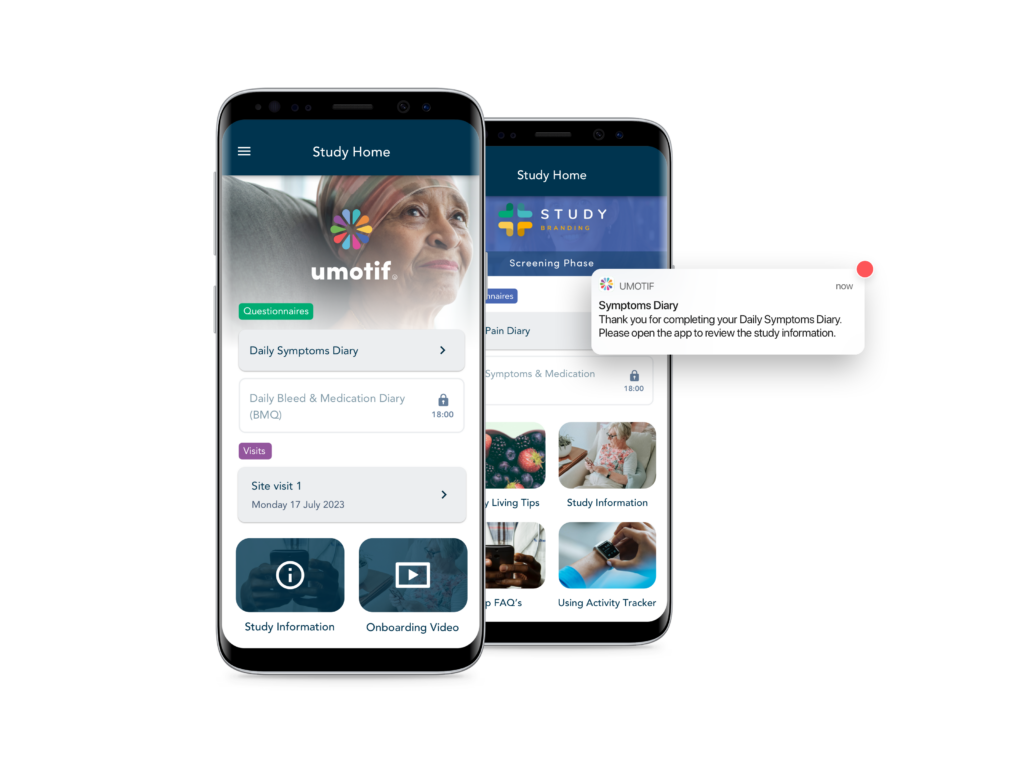
High Compliance
Our patient-centric design combines with flexible alerts and reminders to deliver high data capture compliance. Our eCOA solution regularly achieves >90% compliance in both clinical and real-world studies, helping sponsors run faster, more efficient studies.
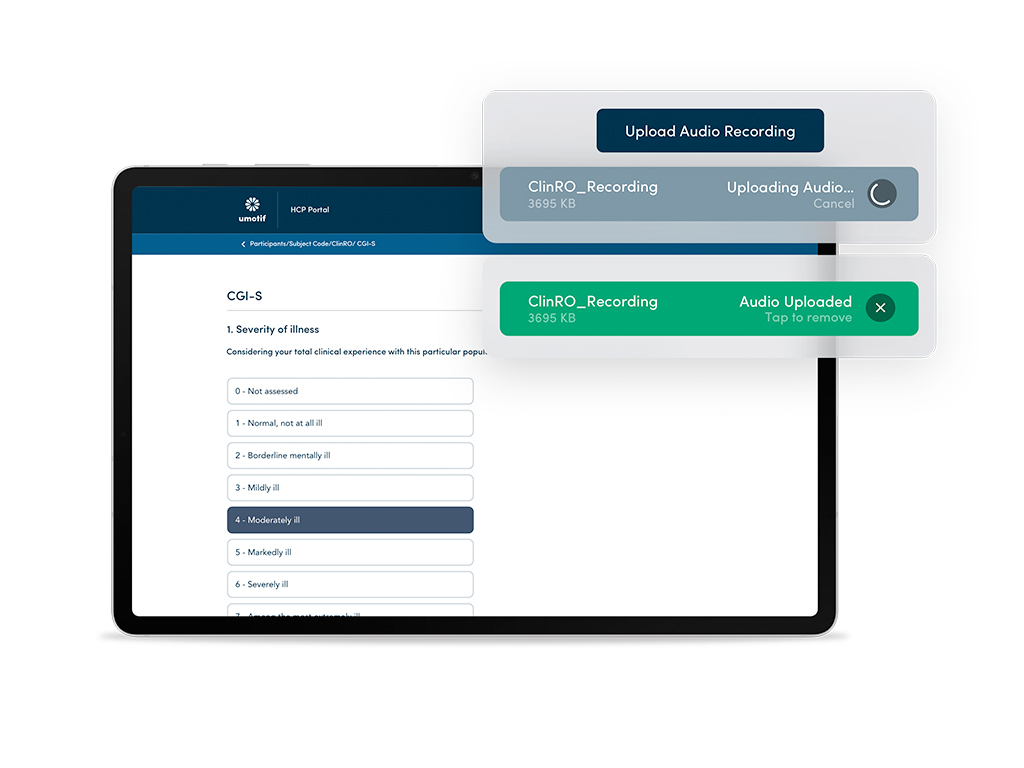
Complex ClinROs and Audio Upload
Capturing complex ClinRO instruments including PANSS, MADRAS, PHQ-9 and HAM-D with audio uploads for Central Rating and Review.
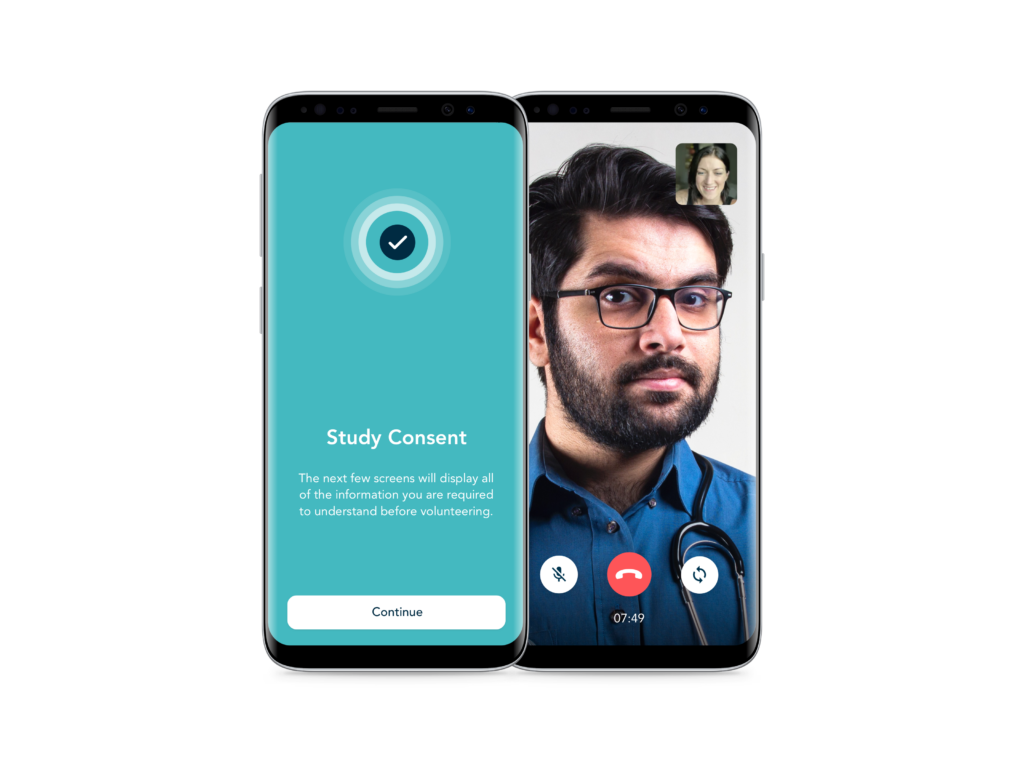
eConsent and Telehealth
Flexible solutions for informed onboarding, consent and telehealth, to enhance the participant experience.
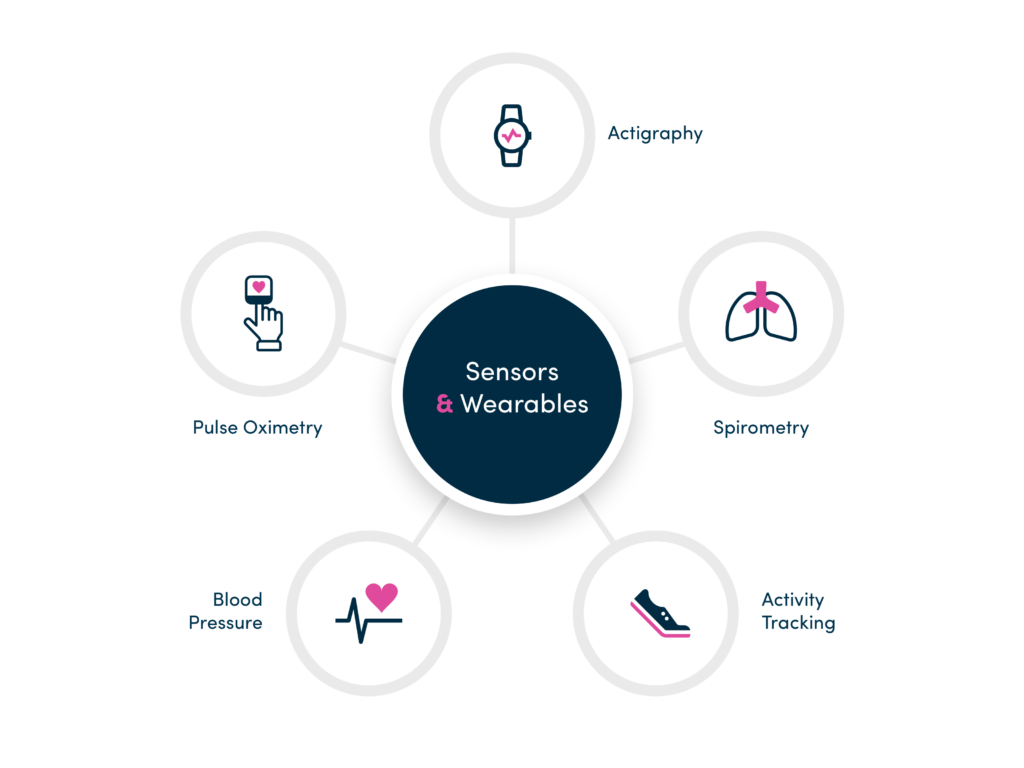
Devices and Wearables
An extensible solution to quickly integrate devices, sensors and 3rd party systems (IVRS, EDC) on a study-by-study basis.
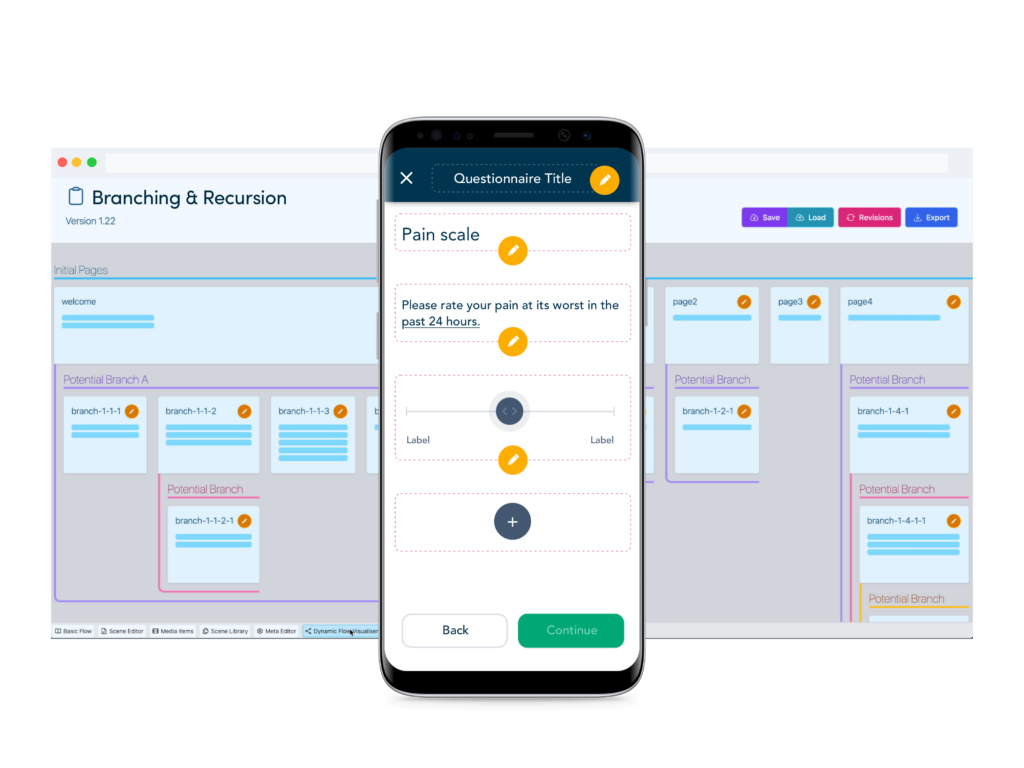
Enhanced questionnaire builder tools
Add validated eCOA instruments and study specific eDiaries with configuration tools designed to rapidly take sponsors from protocol to FPI.
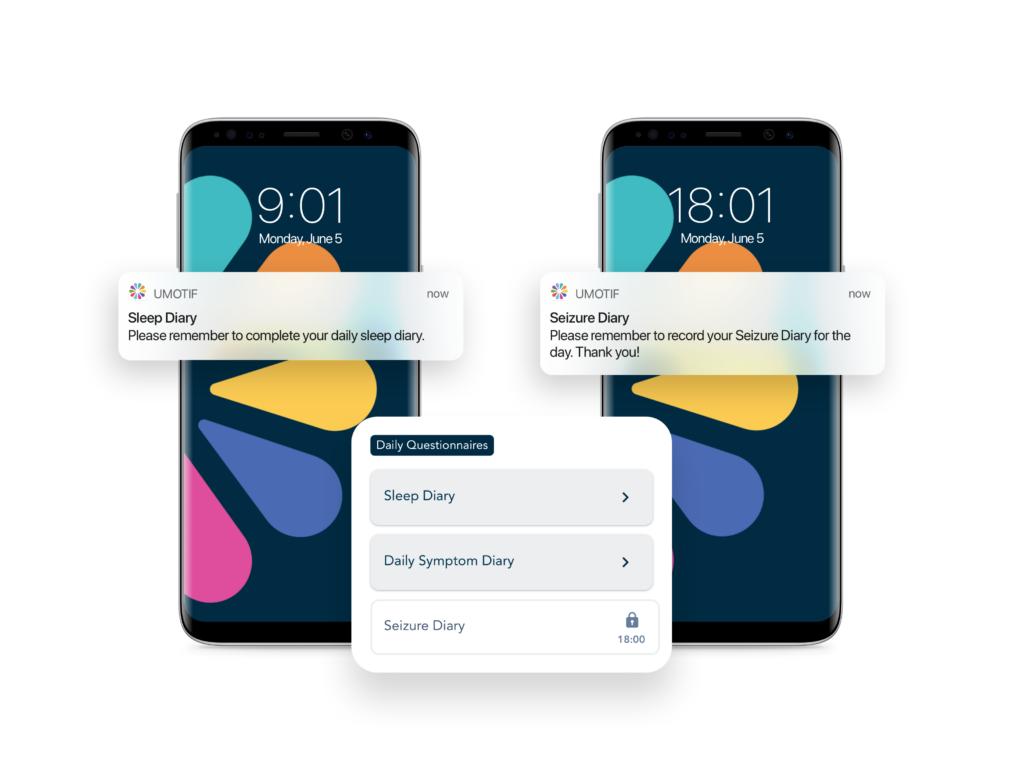
Adaptive eCOA solution scheduling & dynamic alerting
Powerful rules engine and dynamic alerting tools to ensure even the most complex study protocols are robustly implemented, with minimum burden on sites and patients.
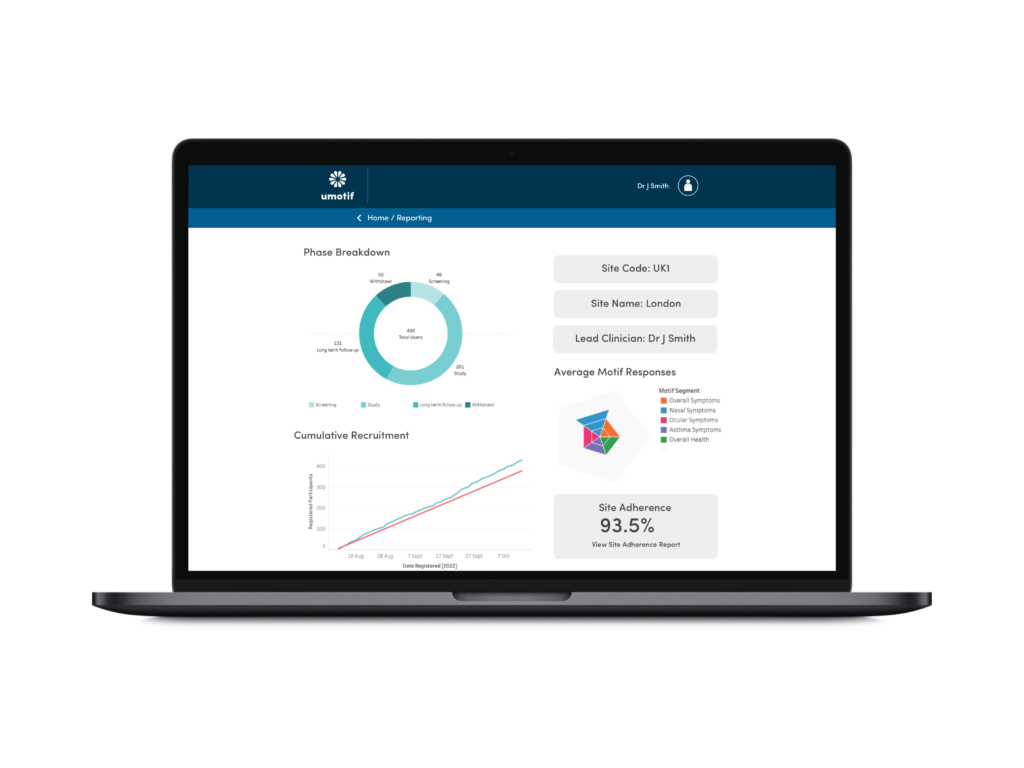
Advanced Reporting and Analytics
Real-time recruitment, eligibility, eCOA compliance and device reporting through a powerful web-based portal to make study management and oversight simpler, faster and easier.

High Compliance
Over 90% compliance in real-world studies

Faster Studies
Reducing study timelines by ⅓

Quality Data
Capturing GCP-compliant data, ready for submission
