Engaged Patients.
Better Trials.
The most modern eConsent and eCOA/ePRO platform powering clinical and real-world research.

We empower the trusted relationship between patient and site for increased recruitment, higher compliance, and fewer dropouts.
The uMotif Advantage
We Start with the Patient.
Patient centricity is the bedrock of modern eCOA systems and the key to delivering a superior participant experience and exceptional data capture.
Since 2012, uMotif’s eCOA platform has been setting a new standard for patient-centric studies — providing a better experience for participants, sites, and sponsors — to drive high compliance and engagement, reduce dropouts, and improve study retention and data completeness.
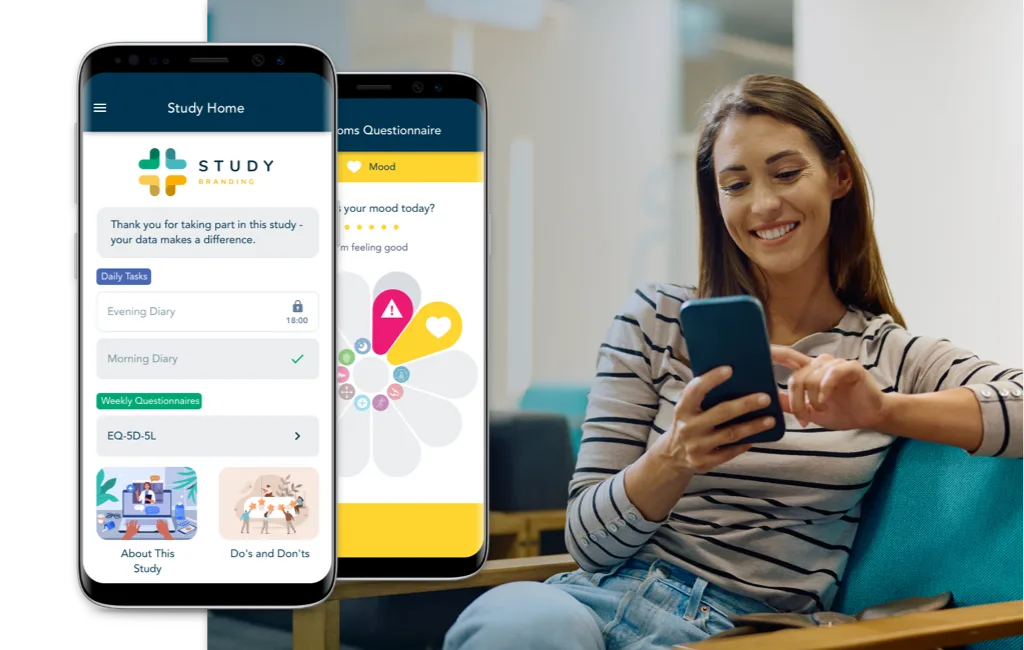
Modern All-in-One eCOA Platform.
uMotif’s cloud native solutions are designed for configurability and scalability to quickly adapt to protocol-specific requirements and provide optimal flexibility for sponsors, sites, and patients.
Our customers are never burdened with legacy technology that does not support modern patient interfaces or lacks tool sets that enable faster and higher quality deployments.
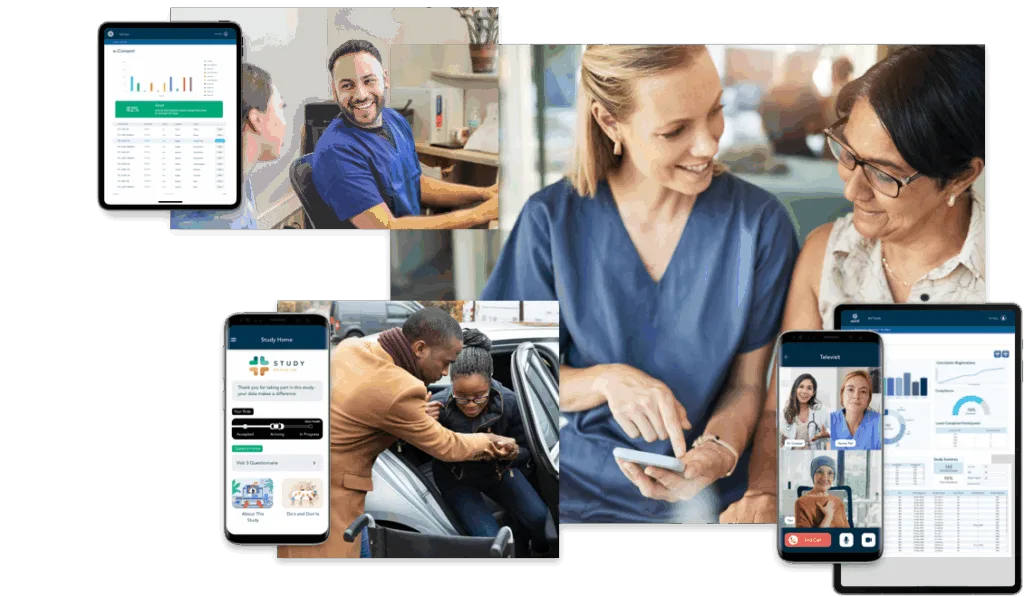
Tools to Bolster the Patient-Site Relationship.
uMotif solutions — including eConsent/Consent Management, Trial Awareness, Patient Ride with Uber Health, and Site Productivity — strengthen the patient-site relationship, which is key to improving eCOA compliance and retention.

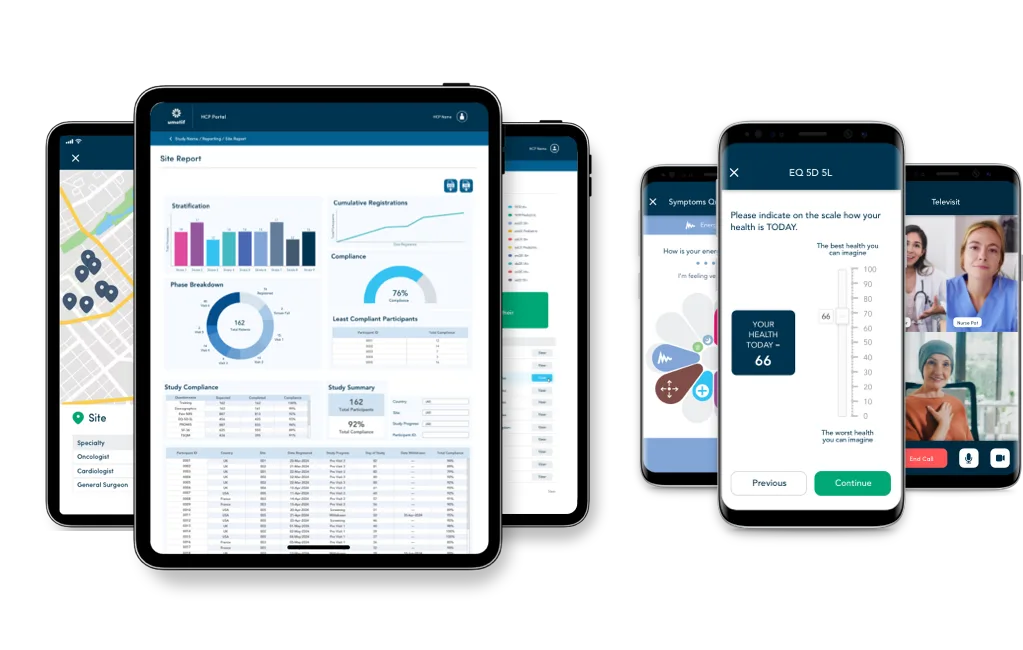
Global. Scalable. Proven.
With cloud-hosting in the US, Europe, and China, the GCP, 21 CFR Part 11 and GDPR-compliant platform supports all study phases and indications.
Real Partnership. Ready to Adapt.
With uMotif, you can expect high-touch support from kickoff to closeout. We work to earn your trust, demonstrate expertise, and communicate with transparency, ensuring that your study is completed on scope, on time, and with the highest quality. We also know that things change, and we’re ready to adapt to your needs.
The uMotif team is led by industry innovators with decades of experience in clinical trials, site operations, technology, and patient engagement. We know what it takes to execute globally and at scale.

In the News
-
uMotif Chief Scientific Officer Leads Publication of Fundamental Work Detailing Industry-Wide Recommendations for Adopting Event-Driven eDiaries in Clinical Trials
Read More -
Partners for Success: Governance Best Practices to Streamline eCOA Trial Launch
Read More -
uMotif Harnesses AI to Deliver Faster, Higher-Quality Clinical Trials with MotifAI Assistant
Read More


