Advancing critical CNS research
Studies in CNS often require the capture of data by clinicians, patients and central raters. uMotif’s robust, flexible and scalable platform powers the capture of ClinROs, ePRO, eDiaries and audio recordings across CNS indications.

Low-burden, robust, highly usable software for CNS studies. Built on the GCP and 21 CFR Part 11 compliant uMotif platform.
Capture complex ClinRO assessments for primary or secondary end points
Built to capture commonly used CNS ClinROs including
- SIGMA/MADRS
- PHQ-9
- C-SSRS
- MoCA
- HAM-D
- Powerful role-based access and protocol-specific scheduling supports the capture of site-based or remote ClinROs for primary and secondary endpoints in CNS studies.
Powering site, remote and home-based data capture for CNS studies
Today’s hybrid study designs include site, at home and remote visits using video conferencing. uMotif’s flexible platform supports all study types – from site-only studies to decentralized designs across multiple countries through our scalable site, participant and central rater video conferencing solutions.

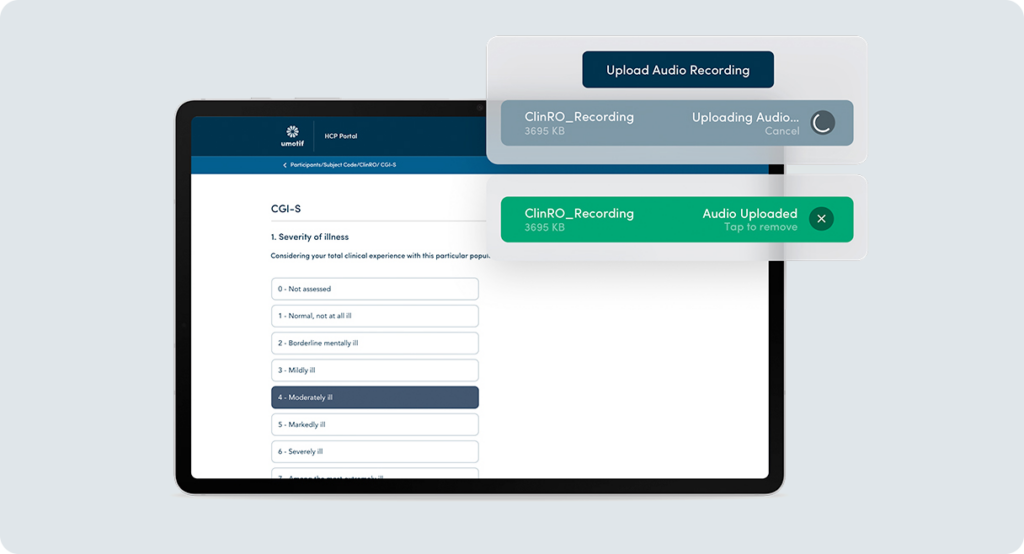
Seamless audio recording for central rating and review
CNS studies often require audio recordings of ClinRO assessments for Central Rating and Review. Whether performed at site or remotely over audio conferencing, uMotif supports the seamless upload of recordings alongside ClinROs for Central Rating and Review processes. Where required, video capture supports developmental studies or for psychedelic study safety.
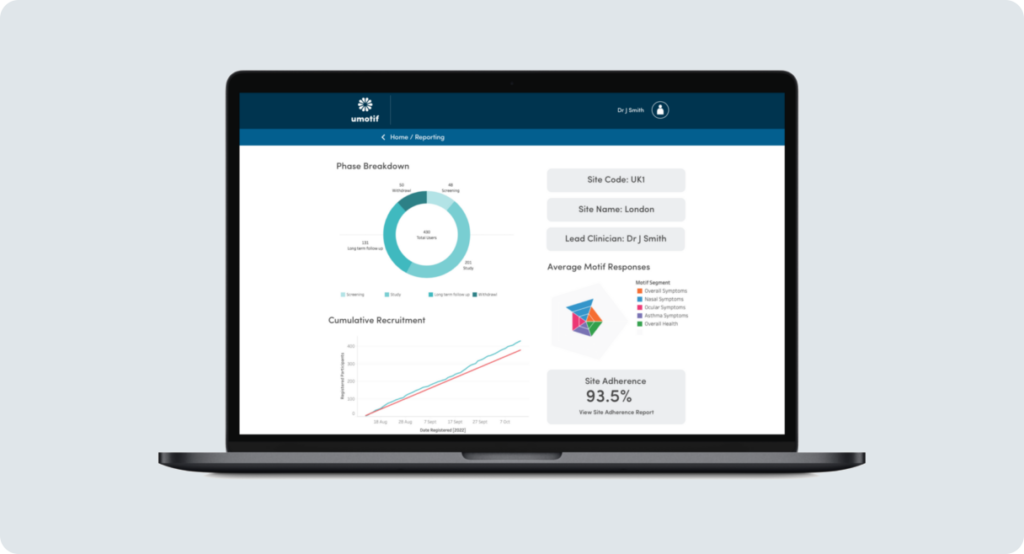
Real-time reporting for faster, more effective study oversight and management
Study sites, CRAs and sponsors need real-time data for more effective and responsive management and oversight. Through uMotif’s role-based reports, study teams get recruitment, compliance and rating data at their fingertips. Combined with alerts and notifications, uMotif’s reporting suite eases the burden of study management.
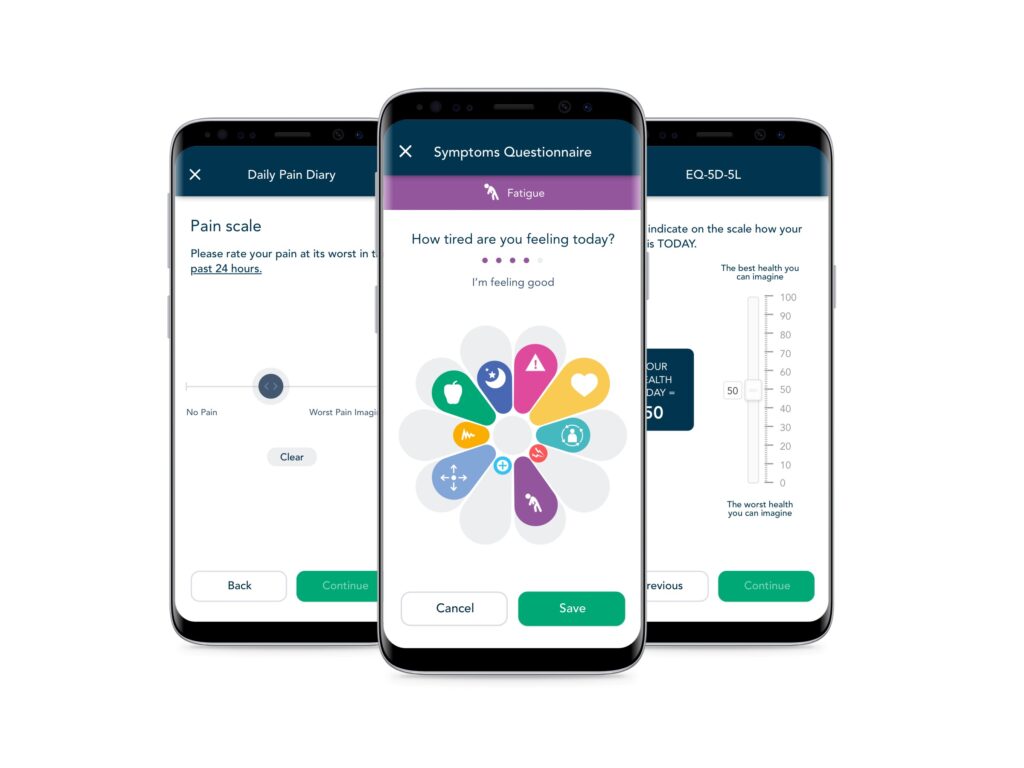
Engaging CNS participants to capture ePRO and eDiaries
Keeping participants engaged, retained and capturing ePRO and eDiary data is critical for study success.
Since 2012, uMotif has been at the forefront of designing solutions that support participants’ clinical trial journey, leading to high levels of engagement and data capture compliance. Our tailored, configurable platform delivers an exceptional patient-centric experience to capture any ePRO/eDiary data for CNS studies.
Expanding CNS research with wearable devices and sensor data
CNS studies increasingly use data from sensors and wearable devices to drive new insights.
uMotif have deep experience in deploying devices to participants to capture additional quantitative data for CNS studies. Our work in Parkinson’s Disease, Chronic Pain and partnerships with Actigraph and others provide sponsors with a tailored ePRO + device solution for CNS studies.