Unrivaled eConsent
uMotif’s Consent Management platform is built to handle the most complex workflows so trials of all designs feel as simple as possible.

Our Consent Management was developed for remote and global workflows – long before the pandemic made decentralized and hybrid trials mainstream.
eConsent
Whether your study is decentralized, hybrid, or fully on-site, our flexible platform ensures every participant, caregiver, and site staff member stays informed and aligned from day one.
Our solution features:
- Fast Deployment: Go live in 2-4 weeks, not months
- Embedded Video eConsent: Include up to 10 stakeholders-patients, caregivers, translators, LARs, and more
- Seamless Integration: Best-in-class API with webhooks for real-time system sync
- Truly Global: Supports any site model with tap-to-join access, print-to-sign capability, and audit-ready reporting.

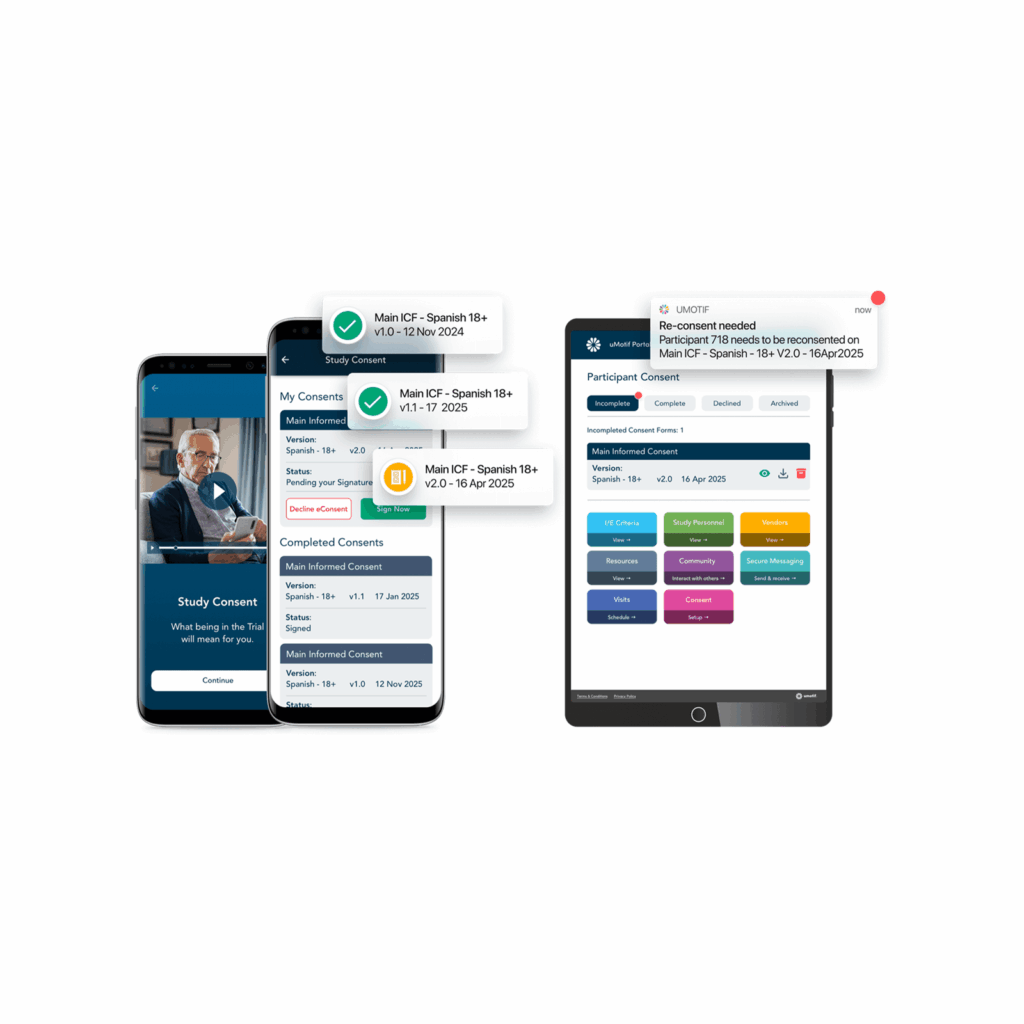
Ongoing Re-Consent
Protocol amendments shouldn’t derail your sites or introduce compliance risks. uMotif ensures version control is bulletproof, keeping the right forms in the right hands—every time.
- Instantly identify patients needing re-consent
- Eliminate versioning errors
- Maintain compliance and avoid audit issues
Data Insights & Monitoring
Consent tracking shouldn’t be a black box. uMotif gives you live visibility across global, country, and site levels—so you can proactively manage timelines, monitor bottlenecks, and improve enrollment outcomes.
