Patient Engagement Solutions
uMotif engages every study participant with our high-touch, configurable experiences that drive >90% compliance in clinical and real-world studies.

Engagement solutions deployed alongside eCOA or standalone supporting all phases of research.
A Companion for Every Trial
Supporting participants through the trial — with their schedule, study information, eCOA, diaries and reminders available through our intuitive and easy-to-use platform to drive high levels of engagement and data capture.


Nudges and Reminders
Timely nudges and dynamic alerts supporting retention and compliance, based on the principles of behavioral psychology.
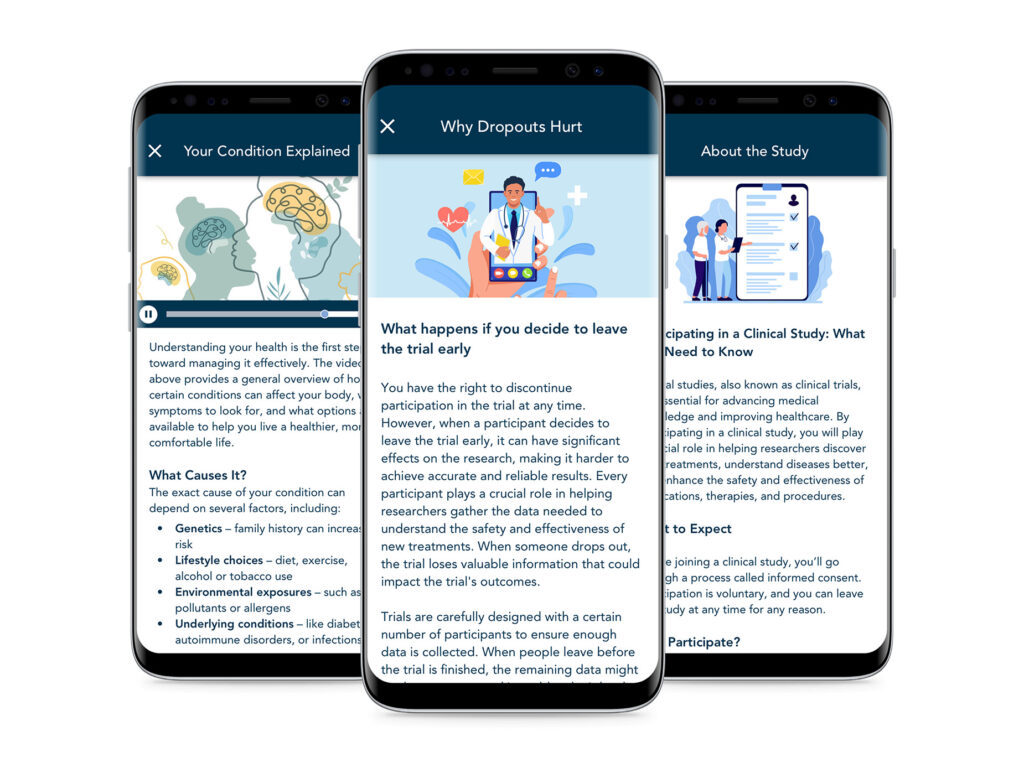
Content and Information
Participants appreciate having study information quickly available to increase trial comprehension; while mid-study updates and educational videos support increased understanding.

Capturing Participant Feedback and Insights
Capturing real-time feedback from participants gives sponsors insights on how the study is performing and can highlight areas for improvement.
Patient Stipends
Through our partners, we support patient stipends based on study activity – helping further engage and retain participants.

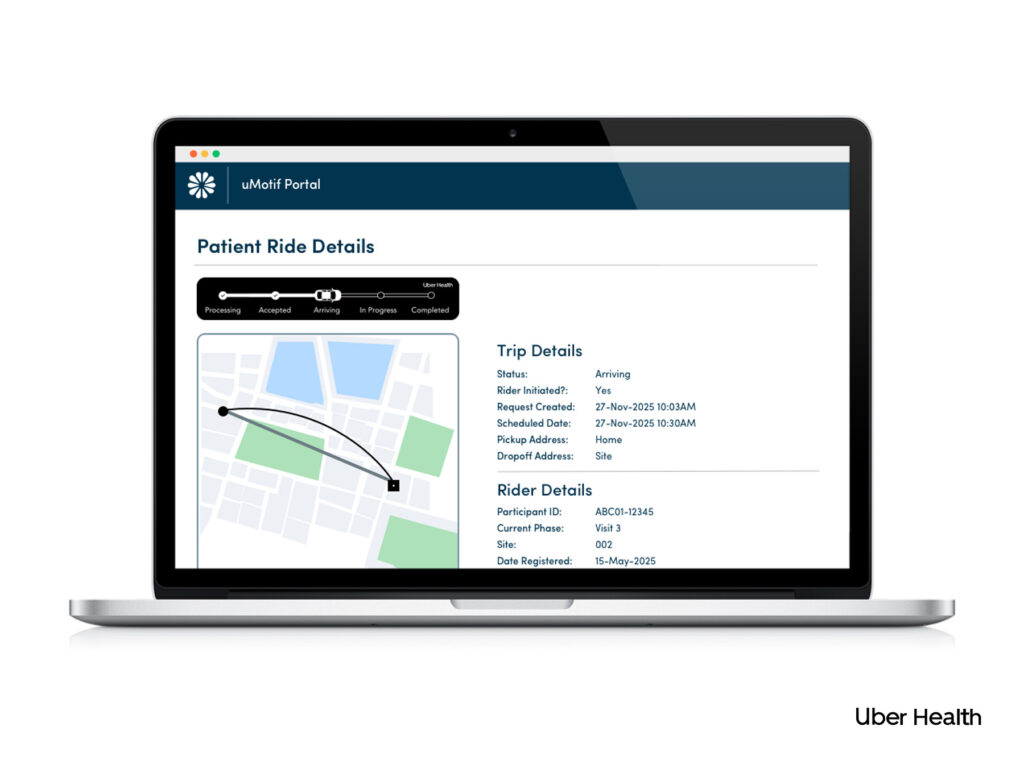
Patient Ride
With Uber Health, sites in the United States can make traveling to visits easier through pre-arranged rides.
Patients and site staff can schedule Uber Health to or from site visits or other approved destinations – all with billing centralized and managed by uMotif within your study budget. This relieves the burden on patients, increases enrollment and retention, and reduces the burden on sites.